Research projects
The research scientist at the Center developed numerous methodologies for the preparation of chemical libraries via solid phase synthesis
as well as liquid phase synthesis, they discovered novel organic compounds with anticancer activity in-vitro and introduced novel
organic derivatives as potential fluorescent labels. One research project is focused on molecular target(s) identification studies with
the main goal to prepare compounds for affinity chromatography. In addition to publications in prestigious scientific journals,
the members of the Center are also authors of several national and international patents.

Novel synthetic routes
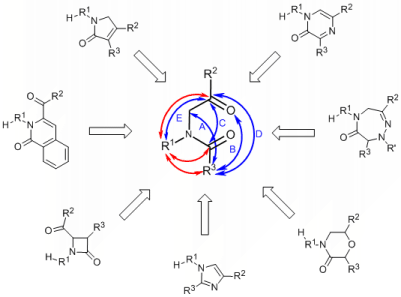 To cover a diverse set of heterocyclic frameworks and at the same time to make syntheses practical, we built individual heterocycles using common intermediates that can be converted to diverse and structurally unrelated heterocycles. This divergent strategy allows us to efficiently prepare compounds with different frameworks and skeletal diversity.
a-Acylamino ketones/esters allow several different ring closure pathways. Blue arrows indicate documented ring formations and the red arrows show the subsequent second ring closures forming the fused ring systems. Synthetic transformations include variety of chemistries (C-C, C=C, C-N, C=N, C-O bond formations) and sizes of heterocycle (4-, 5-, and 6-membered rings) and enabled direct transformation to a range of diverse, structurally unrelated heterocycles including beta-lactams, imidazoles, morpholinones, pyrazinones, dihydro-pyrrol-2-ones, triazepin-6-ones, and isoquinolin-1-ones.1
To cover a diverse set of heterocyclic frameworks and at the same time to make syntheses practical, we built individual heterocycles using common intermediates that can be converted to diverse and structurally unrelated heterocycles. This divergent strategy allows us to efficiently prepare compounds with different frameworks and skeletal diversity.
a-Acylamino ketones/esters allow several different ring closure pathways. Blue arrows indicate documented ring formations and the red arrows show the subsequent second ring closures forming the fused ring systems. Synthetic transformations include variety of chemistries (C-C, C=C, C-N, C=N, C-O bond formations) and sizes of heterocycle (4-, 5-, and 6-membered rings) and enabled direct transformation to a range of diverse, structurally unrelated heterocycles including beta-lactams, imidazoles, morpholinones, pyrazinones, dihydro-pyrrol-2-ones, triazepin-6-ones, and isoquinolin-1-ones.1
Novel synthetic routes
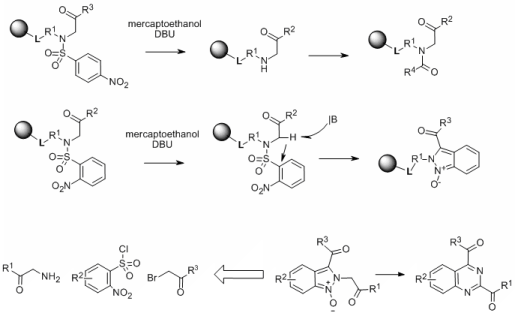 We observed a striking difference in the reaction outcome between 2-Nos and 4-Nos derivatives. Alkylation of resin-bound 2-nitrobenzenesulfonamides by bromoketones and bromoacetates yielded the expected product. However, attempted cleavage of the 2-Nos protecting groups led to unexpected tandem carbon-carbon followed by nitrogen-nitrogen bond formation and yielded indazole oxides of excellent purity.2 On-resin deoxygenation (mesyl chloride/TEA) yielded indazoles.
Interestingly, base-catalyzed rearrangement of 2H-indazoles 1-oxides, prepared from N-alkyl-2-nitro-N-(2-oxo-2-aryl-ethyl)-benzenesulfonamides using glycine, 2-nitrobenzenesulfonyl chlorides and bromo ketones/acetates, yielded high purity quinazolines.3
We observed a striking difference in the reaction outcome between 2-Nos and 4-Nos derivatives. Alkylation of resin-bound 2-nitrobenzenesulfonamides by bromoketones and bromoacetates yielded the expected product. However, attempted cleavage of the 2-Nos protecting groups led to unexpected tandem carbon-carbon followed by nitrogen-nitrogen bond formation and yielded indazole oxides of excellent purity.2 On-resin deoxygenation (mesyl chloride/TEA) yielded indazoles.
Interestingly, base-catalyzed rearrangement of 2H-indazoles 1-oxides, prepared from N-alkyl-2-nitro-N-(2-oxo-2-aryl-ethyl)-benzenesulfonamides using glycine, 2-nitrobenzenesulfonyl chlorides and bromo ketones/acetates, yielded high purity quinazolines.3
Reference List
1. Pudelova, N.; Krchnak, V. J. Comb. Chem. 2009, 11, 851-859.
2. Bouillon, I.; Zajicek, J.; Pudelova, N.; Krchnak, V. J. Org. Chem. 2008, 73, 9027-9032.
3. Krupkova, S.; Slough, G. A.; Krchnak, V. J. Org. Chem. 2010, 75, 4562-4566.

Complexity oriented synthesis: Modulators of protein-protein interactions
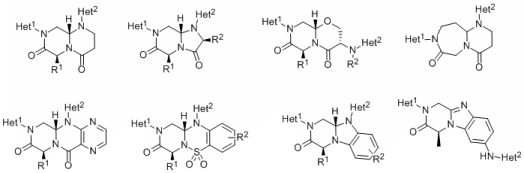 The development of novel small molecules that disrupt protein-protein interactions (PPI) is a key objective in addressing a number of disease states.
PPI occur on a large surface, typically 1,500 - 3,000 Å2, and traditional drug-discovery compound collections have been ineffective in finding compounds capable of disrupting PPI. During the last several years, novel small molecules that target PPI and do not bear any resemblance to natural products have been discovered. We have analyzed structures of known unnatural modulators of these hard targets and designed synthetic routes to access structurally convoluted molecules that differ from traditional drug-like compounds. In this project we intend to design and synthesize compounds with a potential to disrupt PPI characterized by a complex framework composed of two different heterocycles attached to a range of fused or bridged bicyclic cores.
The bicyclic core unit will serves as a conformationally constrained scaffold with spatially defined locations of two heterocyclic moieties. The acyclic precursors of the bicyclic core units will be assembled from simple building blocks in a step-wise manner on solid phase. Following assembly of the acyclic precursor, two heterocycles will be synthesized in a combinatorial manner using suitably functionalized acyclic core. Synthetic routes will be designed to facilitate cyclization to the core unit either still attached to the resin or concurrently with the release of target compounds from the resin.
In summary, the overall objective of this project is to expand our knowledge of interactions of proteins with fully synthetic small organic molecules and initiate a systematic study aimed at understanding disruption of PPI by synthetic molecules.
The development of novel small molecules that disrupt protein-protein interactions (PPI) is a key objective in addressing a number of disease states.
PPI occur on a large surface, typically 1,500 - 3,000 Å2, and traditional drug-discovery compound collections have been ineffective in finding compounds capable of disrupting PPI. During the last several years, novel small molecules that target PPI and do not bear any resemblance to natural products have been discovered. We have analyzed structures of known unnatural modulators of these hard targets and designed synthetic routes to access structurally convoluted molecules that differ from traditional drug-like compounds. In this project we intend to design and synthesize compounds with a potential to disrupt PPI characterized by a complex framework composed of two different heterocycles attached to a range of fused or bridged bicyclic cores.
The bicyclic core unit will serves as a conformationally constrained scaffold with spatially defined locations of two heterocyclic moieties. The acyclic precursors of the bicyclic core units will be assembled from simple building blocks in a step-wise manner on solid phase. Following assembly of the acyclic precursor, two heterocycles will be synthesized in a combinatorial manner using suitably functionalized acyclic core. Synthetic routes will be designed to facilitate cyclization to the core unit either still attached to the resin or concurrently with the release of target compounds from the resin.
In summary, the overall objective of this project is to expand our knowledge of interactions of proteins with fully synthetic small organic molecules and initiate a systematic study aimed at understanding disruption of PPI by synthetic molecules.

DOS of tricyclic fused bisheterocycles
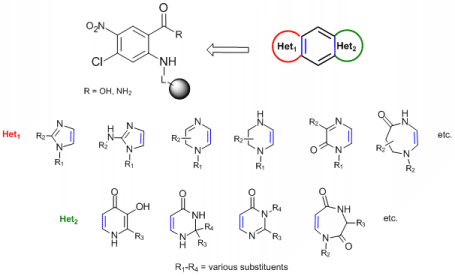 Diversity-Orientated Synthesis (DOS) and Target-Orientated Synthesis (TOS) are the most powerful synthetic strategies frequently used for identification of new biologically active compounds as primar structures (hits) intended for further development.1 One of the crucial tasks for development of an efficient DOS is achievement of high skeletal diversity of the target molecules.
Heterocyclic compounds represent a group of organic molecules with high biological potential which can be displayed by number of heterocycles used in a current human therapy. An inherent subgroup of pharmacologicaly relevant heterocycles includes compounds that contain two heterocyclic units, i.e. bisheterocycles (BHs).2 BHs have not been studied extensively in comparison to single heterocycles and can be divided to two major groups: Nonrigid systems (heterocyclic skeletons connected via spacer) and rigid (condensed, fused) derivatives, which can act (in favourable size and configuration) as DNA intercalators.
General objective of this project is development of DOS of highly diverse tricyclic fused BHs. An example of the target molecules is preparation of [x+6+y] molecules given below. Letters x and y represent nitrogenous heterocyclic scaffolds of various size/substitution situated at the opposite sides of a benzene ring (see Figure). Formation of Het1 takes advantage of methods described for modification of o-phenylendiamines.3 Synthesis of Het2 will be tested with use of strategies developed for modification of anthranilic acids.4-7 The preparation of the target compounds will be performed with use of solid-phase synthesis in combinatorial fashion. For instance, target heterocycles displayed below will in theory afford 24 different BHs (in an absolute number of combinations), each BH with 4 diverse positions.
The chemical libraries obtained will be subjected to High Throughput Screening (HTS) and selected hits will be further optimized with use of TOS. Alternatively, the development process will be performed without HTS on representative cancer cell lines according to the previously described approach.8 Selected derivatives will be subjected to study of their fluorescence properties to explore their possible use as fluorescence labels.9
Diversity-Orientated Synthesis (DOS) and Target-Orientated Synthesis (TOS) are the most powerful synthetic strategies frequently used for identification of new biologically active compounds as primar structures (hits) intended for further development.1 One of the crucial tasks for development of an efficient DOS is achievement of high skeletal diversity of the target molecules.
Heterocyclic compounds represent a group of organic molecules with high biological potential which can be displayed by number of heterocycles used in a current human therapy. An inherent subgroup of pharmacologicaly relevant heterocycles includes compounds that contain two heterocyclic units, i.e. bisheterocycles (BHs).2 BHs have not been studied extensively in comparison to single heterocycles and can be divided to two major groups: Nonrigid systems (heterocyclic skeletons connected via spacer) and rigid (condensed, fused) derivatives, which can act (in favourable size and configuration) as DNA intercalators.
General objective of this project is development of DOS of highly diverse tricyclic fused BHs. An example of the target molecules is preparation of [x+6+y] molecules given below. Letters x and y represent nitrogenous heterocyclic scaffolds of various size/substitution situated at the opposite sides of a benzene ring (see Figure). Formation of Het1 takes advantage of methods described for modification of o-phenylendiamines.3 Synthesis of Het2 will be tested with use of strategies developed for modification of anthranilic acids.4-7 The preparation of the target compounds will be performed with use of solid-phase synthesis in combinatorial fashion. For instance, target heterocycles displayed below will in theory afford 24 different BHs (in an absolute number of combinations), each BH with 4 diverse positions.
The chemical libraries obtained will be subjected to High Throughput Screening (HTS) and selected hits will be further optimized with use of TOS. Alternatively, the development process will be performed without HTS on representative cancer cell lines according to the previously described approach.8 Selected derivatives will be subjected to study of their fluorescence properties to explore their possible use as fluorescence labels.9
Reference List
1. Schreiber, S. L. Science 2000, 287 (5460), 1964-1969.
2. Soural, M.; Bouillon, I.; Krchnak, V. J.Comb.Chem. 2008, 10, 923-933.
3. Krchnak, V.; Smith, J.; Vagner, J. Collect. Czech. Chem.Commun. 2001, 66, 1078-1106.
4. Krupkova S.; Soural M.; Hlavac J.; Hradil P., J.Comb.Chem. 2009, 11, 951-955
5. Soural, M.; Krchnak, V., J.Comb.Chem. 2007, 9, 793-796.
6. Soural M.; Funk P.; Kvapil L.; Hradil P.; Hlavac J.; Bertolasi V. Arkivoc 2010, 10, 255-265.
7. Boojamra C. G.; Burow, K. M.; Thompson L.A.; Ellman J. A. J.Org.Chem. 1997, 62, 1240-1256
8. Soural, M.; Hlavac, J.; Funk, P.; Dzubak, P.; Hajduch, M. ACS Comb.Sci. 2011, 13, 39-44..
9. Motyka, K.; Hlavac, J.; Soural, M.; Funk, P. Tetrahedron Lett. 2010, 51, 5060-5063.

Synthesis of bisheterocyclic systems including nucleic acid component in their structure
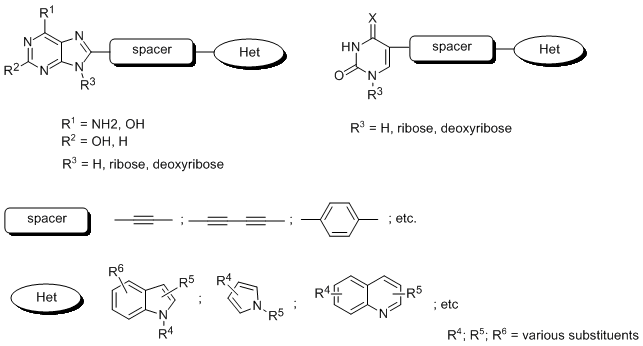 Study of modified nucleic acid components is one of the successful development course leading to products successfully used in medical treatment or nucleic acid structural studies. Several derivatives of this structure are known especially for their anticancer activity (5-fluorouracil, gemcitabine, etc.) and antiviral activity (cidofovir, tenofovir, etc.). The mechanism of their action is based on inhibition of some important enzymes, like kinases, polymerases, reverse transcriptases, etc. or termination of polymerization process leading to nucleic acid or changing the nucleic acid structure and function.
In our present research we are interested in use of combinatorial chemistry for preparation of chemical libraries of variously substituted pyrimidine1,2 or purine3 heterocycles destined for study of their anticancer activity. For recognition of structure-activity relationship method of generic chemical libraries synthesis and testing was developed4. Recently we have turned our attention to development of new fluorophoric systems, potentially used as molecular probes5,6.
The work will be focused on development of systems including natural nucleoside or nucleotide in their molecule (see figure 1) with use of combinatorial chemistry approach and its incorporation to oligonucleotides via automated synthesis.
Study of modified nucleic acid components is one of the successful development course leading to products successfully used in medical treatment or nucleic acid structural studies. Several derivatives of this structure are known especially for their anticancer activity (5-fluorouracil, gemcitabine, etc.) and antiviral activity (cidofovir, tenofovir, etc.). The mechanism of their action is based on inhibition of some important enzymes, like kinases, polymerases, reverse transcriptases, etc. or termination of polymerization process leading to nucleic acid or changing the nucleic acid structure and function.
In our present research we are interested in use of combinatorial chemistry for preparation of chemical libraries of variously substituted pyrimidine1,2 or purine3 heterocycles destined for study of their anticancer activity. For recognition of structure-activity relationship method of generic chemical libraries synthesis and testing was developed4. Recently we have turned our attention to development of new fluorophoric systems, potentially used as molecular probes5,6.
The work will be focused on development of systems including natural nucleoside or nucleotide in their molecule (see figure 1) with use of combinatorial chemistry approach and its incorporation to oligonucleotides via automated synthesis.
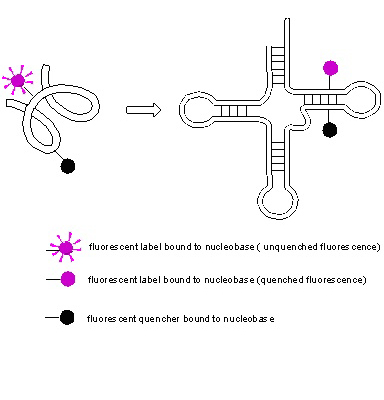 The compounds will be tested for potential anticancer activity and specific enzyme activity inhibition (like DNA, RNA polymerase inhibition, CDK inhibition, etc.)
The compounds will be also tested for their fluorescence properties (lighting/quenching) to select suitable labels of nucleic acids. Incorporation of labeled nucleotides to nucleic acid sequences in combination with techniques as FRET (Fluorescence Resonance Energy Transfer) will be studied in context of potential use of these compounds as nucleic acid conformation analysis tool (see figure 2)
The fluorescence properties will be studied also under various pH to select potential intracellular pH probes and in presence of various biogenic metal ionts, like Ca2+, Mg2+ to select potential indicators of these ions in intracellular matrix.
The compounds will be tested for potential anticancer activity and specific enzyme activity inhibition (like DNA, RNA polymerase inhibition, CDK inhibition, etc.)
The compounds will be also tested for their fluorescence properties (lighting/quenching) to select suitable labels of nucleic acids. Incorporation of labeled nucleotides to nucleic acid sequences in combination with techniques as FRET (Fluorescence Resonance Energy Transfer) will be studied in context of potential use of these compounds as nucleic acid conformation analysis tool (see figure 2)
The fluorescence properties will be studied also under various pH to select potential intracellular pH probes and in presence of various biogenic metal ionts, like Ca2+, Mg2+ to select potential indicators of these ions in intracellular matrix.
Reference List
1. Brulikova, L.; Dzubak, P.; Hajduch, M.; Lachnitova, L.; Kollareddy, M.; Kolar, M.; Bogdanova, K.; Hlavac, J. Eur.J.Med.Chem. 2010, 45, 3588-3594.
2. Spacilova, L.; Dzubak, P.; Hajduch, M.; Krupkova, S.; Hradil, P.; Hlavac, J. Bioorg.Med.Chem.Lett. 2007, 17, 6647-6650.
3. Vankova, B.; Hlavac, J.; Soural, M. J.Comb.Chem. 2010, 12, 890-894.
4. Soural, M.; Hlavac, J.; Funk, P.; Dzubak, P.; Hajduch, M. ACS Comb.Sci. 2011, 13, 39-44..
5. Motyka, K.; Hlavac J.; Soural, M.; Funk, P. Tetrahedron Lett. 2010, 51, 5060-5063
6. Motyka, K.; Hlavac, J.; Soural, M.; Hradil, P.; Krejci, P.; Kvapil, L.; Weiss, M. Tetrahedron Lett. 2011, 52, 715-717.
